Author: Declan Frobisher - Page 2
Pregnancy Trimester-Specific Medication Risks: Safer Timing Strategies
- 3.02.2026
- Posted in Health
- 8 Comments

Learn how medication risks change during each trimester of pregnancy. Discover which drugs are safest when, how to avoid birth defects, and what to do if you took something before knowing you were pregnant.
Steroids and NSAIDs Together: Why This Combo Raises GI Bleeding Risk and How to Stop It
- 2.02.2026
- Posted in Health
- 9 Comments
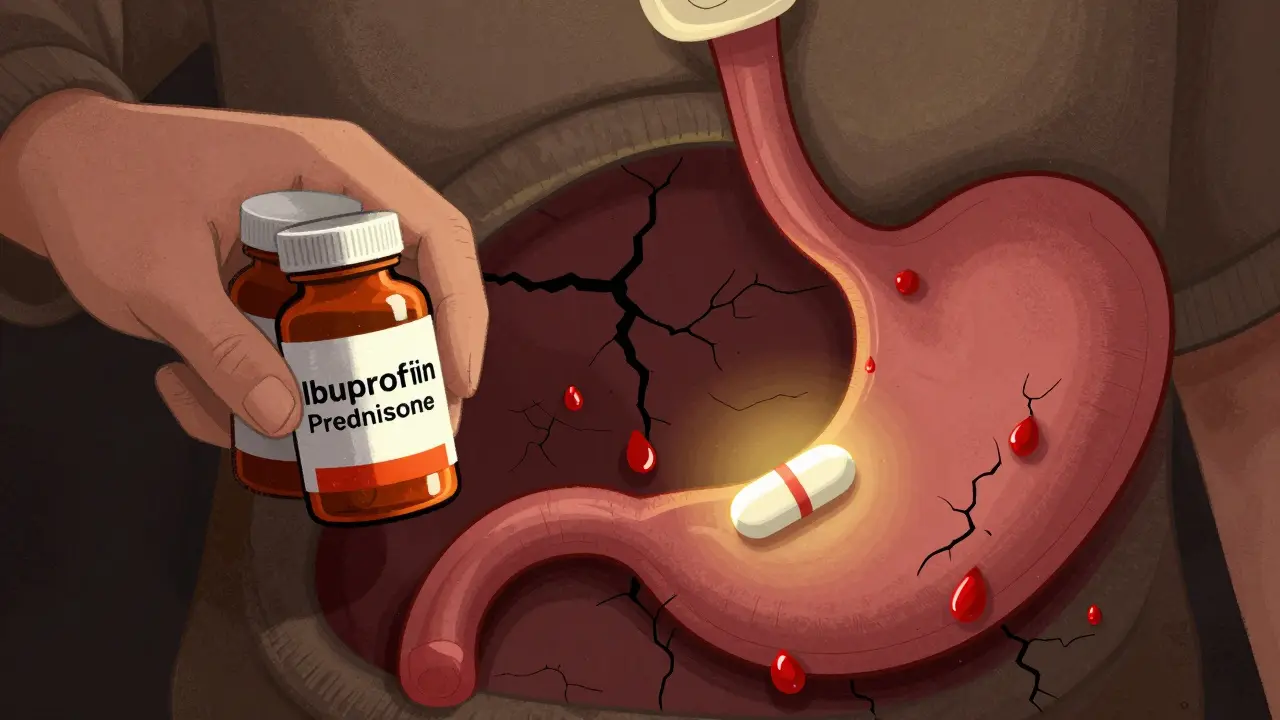
Combining steroids and NSAIDs can raise the risk of dangerous GI bleeding by up to 12 times. Learn why this happens, who's most at risk, and how to prevent it with proven, simple steps.
Rheumatoid Arthritis: How Biologic DMARDs Can Lead to Disease Remission
- 1.02.2026
- Posted in Health
- 10 Comments
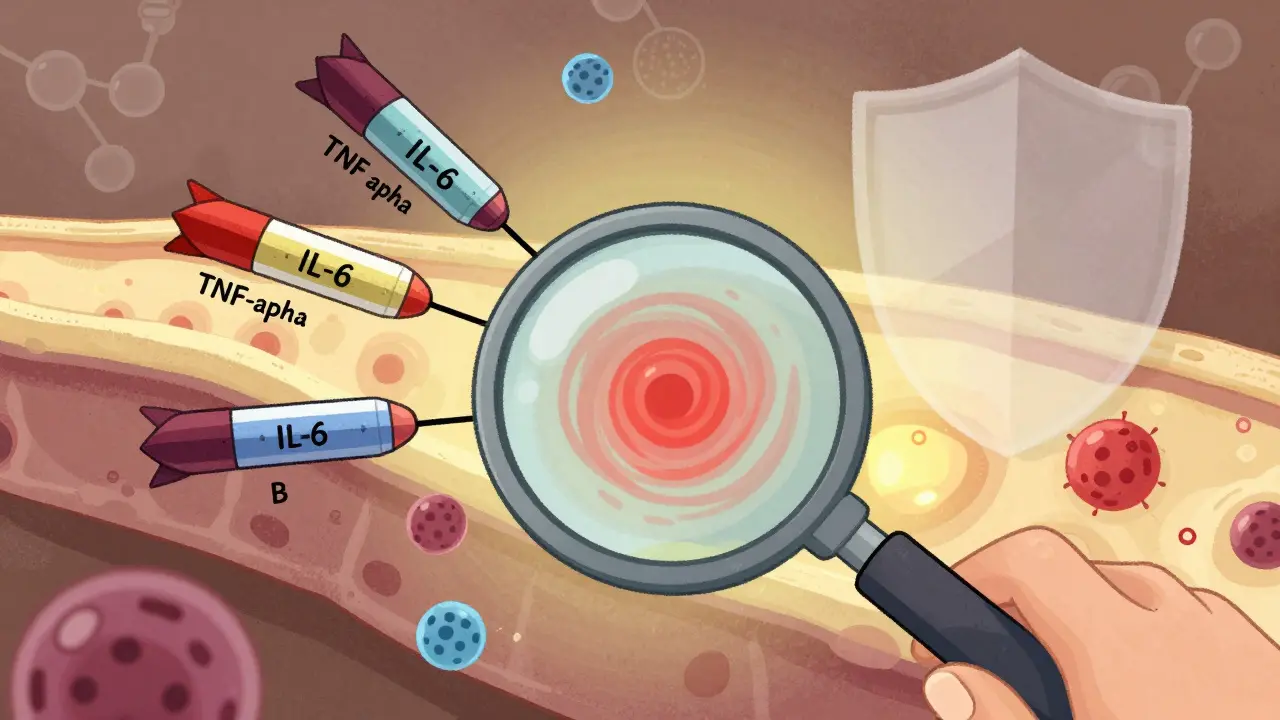
Biologic DMARDs have transformed rheumatoid arthritis treatment, offering real remission for many patients. Learn how these targeted therapies work, which ones are most effective, and what to expect from treatment.
Which Statins Cause the Most Muscle Pain? Real Data on Risk and Relief
- 31.01.2026
- Posted in Health
- 13 Comments

Most muscle pain blamed on statins isn't actually caused by them. Learn which statins carry the highest risk, how to tell if it's the drug or your brain, and what to do if you're worried about side effects.
How the FDA Monitors Generic Drug Safety After Approval
- 30.01.2026
- Posted in Health
- 9 Comments

The FDA doesn't stop monitoring generic drugs after approval. It uses millions of reports, advanced data tools, and targeted watch lists to catch manufacturing flaws and rare safety issues. Here's how it works-and where gaps remain.
Mental Health Medications in Pregnancy: What You Need to Know About Shared Decision-Making
- 28.01.2026
- Posted in Health
- 12 Comments

Learn how shared decision-making helps pregnant people safely manage mental health medications, balancing risks of untreated illness against medication safety. Evidence-based guidance on SSRIs, lithium, and alternatives.
Measuring Your Medication Adherence: A Practical Checklist
- 27.01.2026
- Posted in Health
- 14 Comments

Learn how to track your medication adherence with a simple, practical checklist. Understand real metrics like PDC, avoid common mistakes, and find easy ways to stay on track with your prescriptions.
SNRI Medications: Extended Treatment Options for Mental Health
- 26.01.2026
- Posted in Health
- 16 Comments
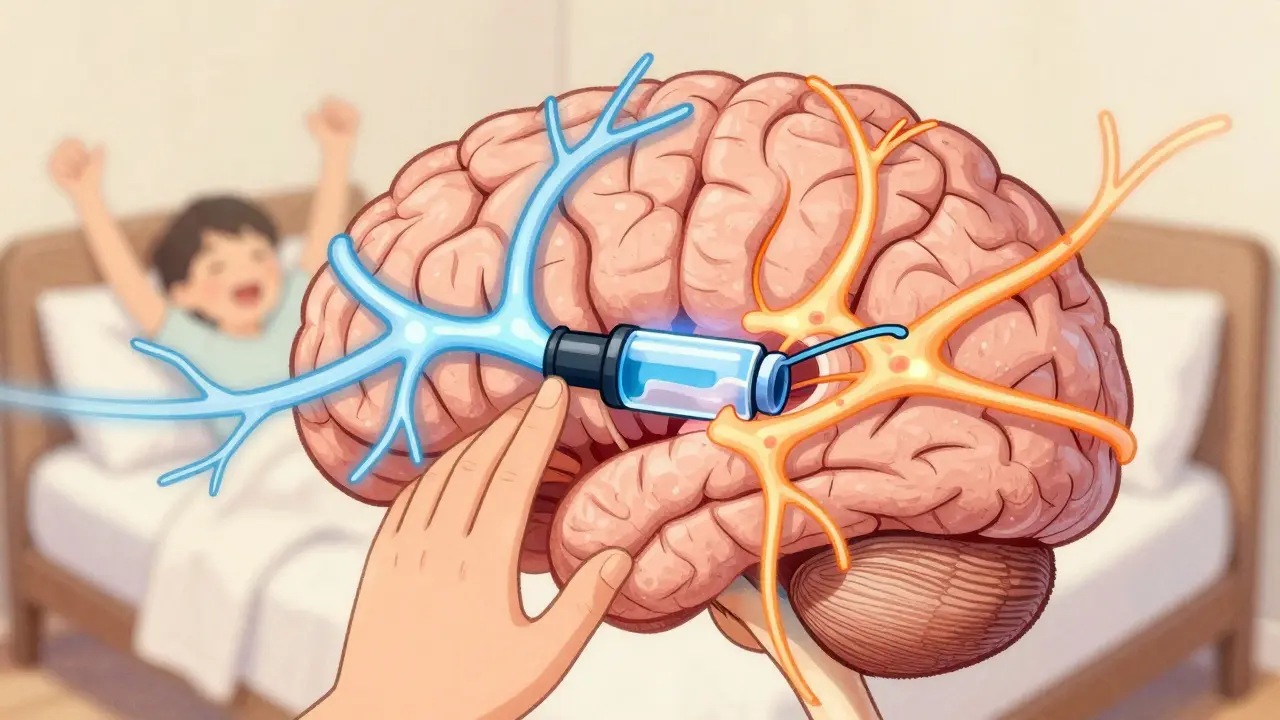
SNRI medications offer a dual-action approach to treating depression, anxiety, and chronic pain by boosting serotonin and norepinephrine. Learn how they work, who benefits most, and what to expect from side effects and withdrawal.
Proton Pump Inhibitors with Antiplatelets: How to Reduce GI Bleed Risk Without Compromising Heart Protection
- 26.01.2026
- Posted in Health
- 10 Comments
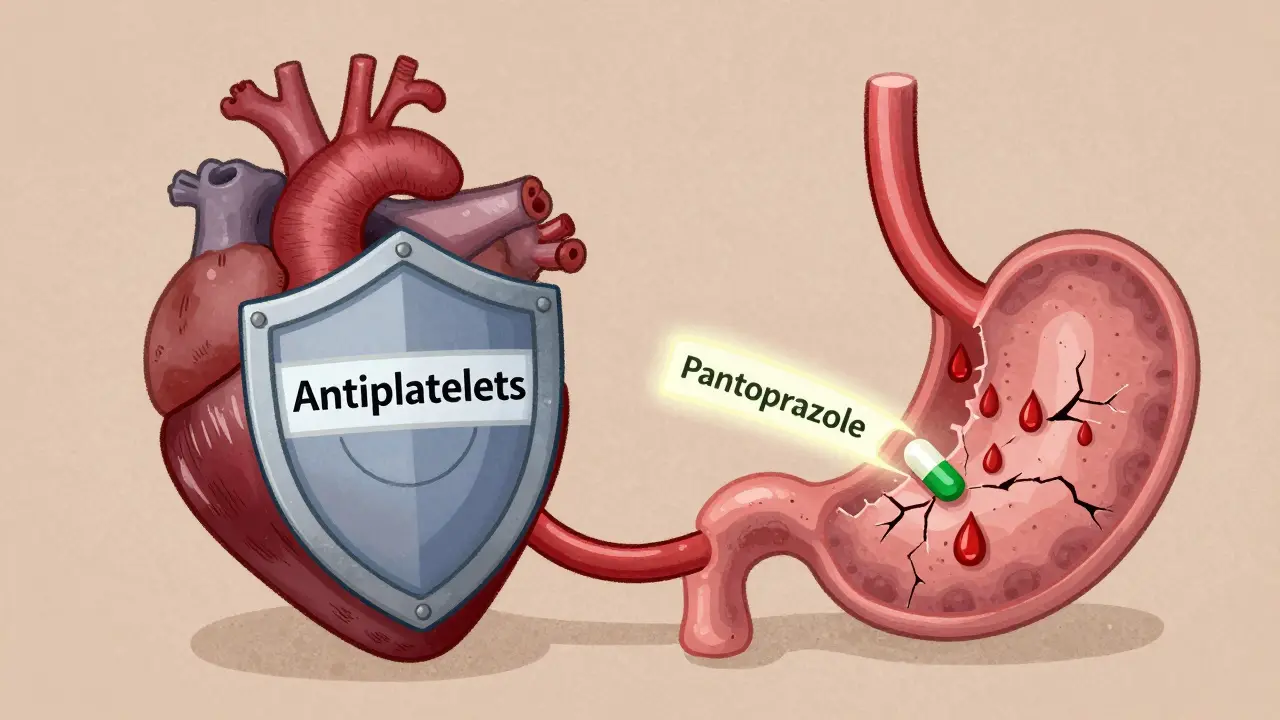
Proton pump inhibitors reduce GI bleeding risk in patients on antiplatelet therapy by up to 37%. Learn which PPIs are safe with clopidogrel, when to use them, and how to avoid unnecessary long-term use.
Key Medication Safety Terms Patients Should Know and Use
- 24.01.2026
- Posted in Health
- 9 Comments

Learn the essential medication safety terms every patient should know-from the Eight Rights to adverse drug events-to prevent harmful errors and take control of your health.
Switching to an Authorized Generic: How to Manage Patient Transitions Smoothly
- 23.01.2026
- Posted in Health
- 14 Comments

Authorized generics offer the same medication as brand-name drugs at lower prices, with fewer side effects. Learn how to safely switch patients, check insurance coverage, and avoid common pitfalls in medication transitions.
Herpes Simplex Virus: Types, Symptoms, and Antiviral Therapy
- 22.01.2026
- Posted in Health
- 10 Comments
Herpes Simplex Virus has two main types: HSV-1 and HSV-2. Learn how they differ in symptoms, transmission, and treatment. Antiviral therapy can reduce outbreaks and prevent spread - even without visible sores.

