Tag: bioequivalence
Partial AUC in Bioequivalence: How Advanced Metrics Ensure Drug Safety and Efficacy
- 14.01.2026
- Posted in Health
- 8 Comments
Partial AUC (pAUC) is a sophisticated pharmacokinetic tool used by the FDA and EMA to ensure generic drugs match brand-name versions in early drug release-critical for safety and efficacy. Learn how it works, why it's replacing old metrics, and what it means for generic drug development.
How to Compare Dissolution Profiles and What They Mean for Generic Drugs
- 28.12.2025
- Posted in Health
- 11 Comments

Dissolution profile comparison is the key method regulators use to prove generic drugs work like brand-name versions. Learn how f2 scores, lab standards, and advanced stats ensure generics are safe and effective.
Modified-Release Formulations: Key Bioequivalence Rules You Need to Know
- 15.12.2025
- Posted in Health
- 11 Comments
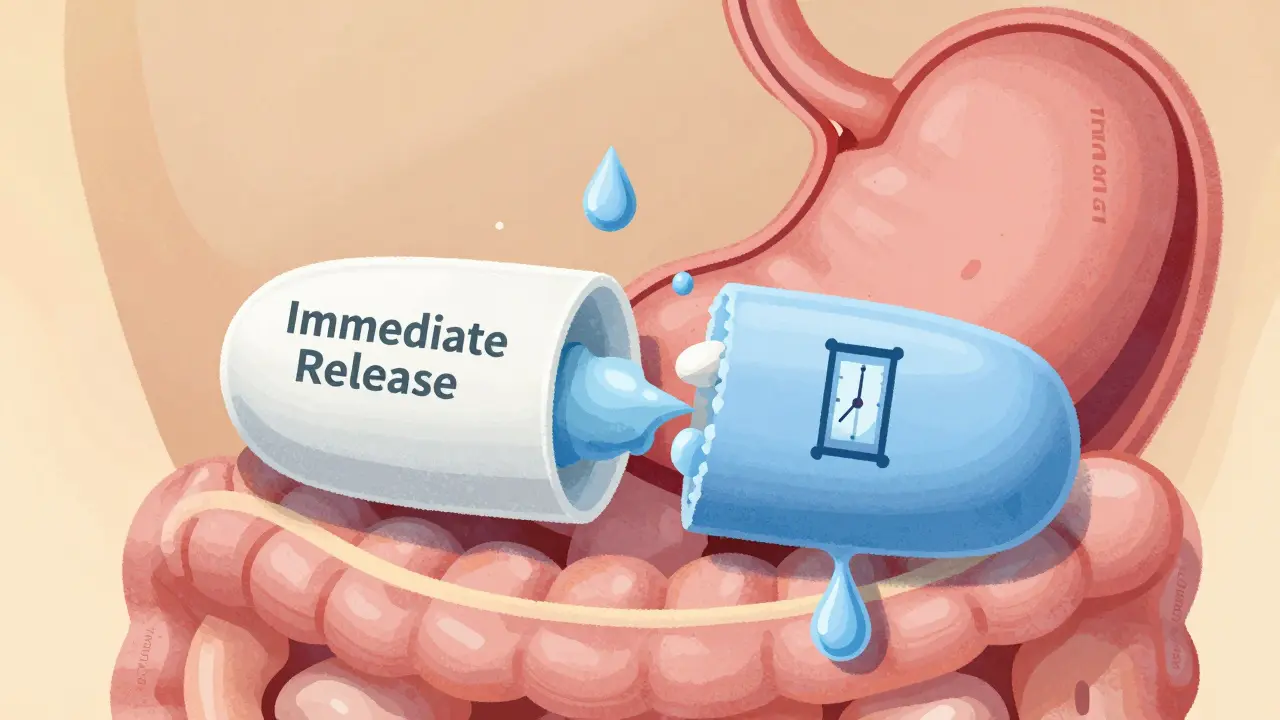
Modified-release formulations require special bioequivalence testing to ensure generic versions release drugs at the same rate and time as brand-name drugs. Learn the key regulatory requirements, testing methods, and common failure points.
Switching Between Generic Medications: What You Need to Know
- 5.12.2025
- Posted in Health
- 9 Comments
Switching between different generic medications can be safe - or risky, depending on the drug. Learn which generics are interchangeable, which ones need caution, and how to protect yourself from dangerous changes.

