Tag: bioequivalence studies
Modified-Release Formulations: Key Bioequivalence Rules You Need to Know
- 15.12.2025
- Posted in Health
- 11 Comments
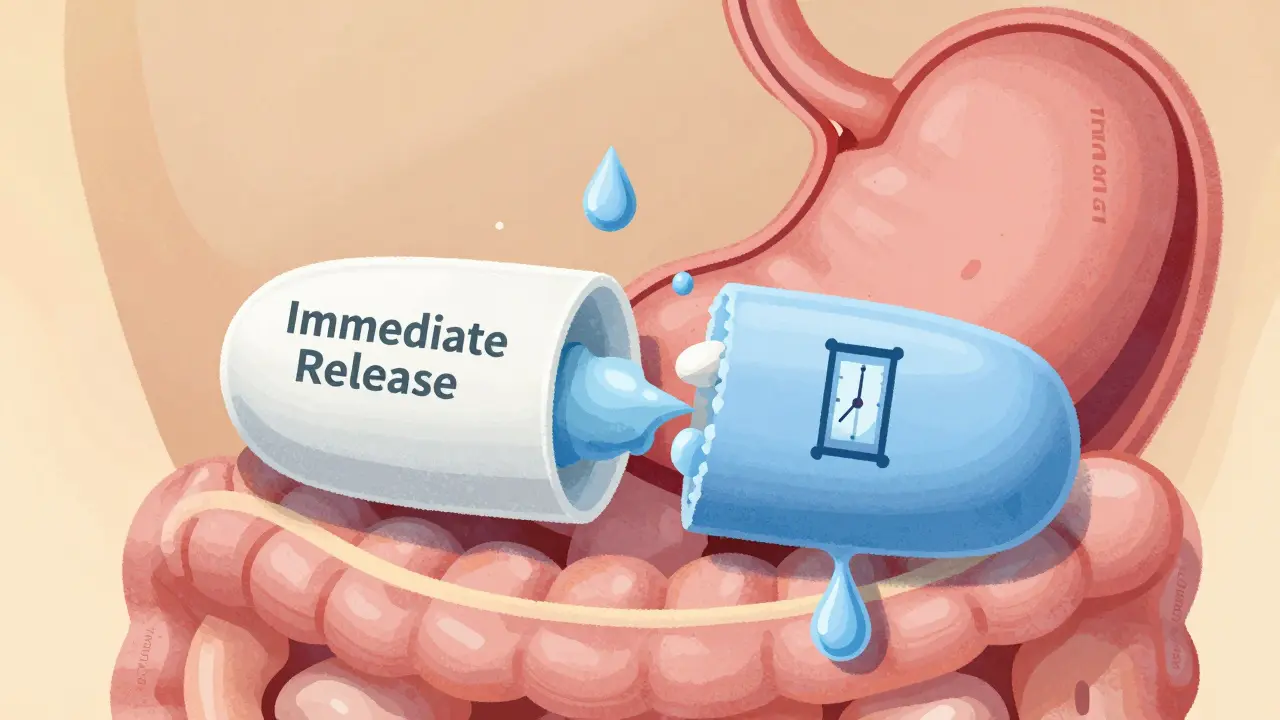
Modified-release formulations require special bioequivalence testing to ensure generic versions release drugs at the same rate and time as brand-name drugs. Learn the key regulatory requirements, testing methods, and common failure points.

