When a factory produces a batch of medical devices and 12 out of 500 units fail inspection, what happens next? Many assume someone just throws out the bad ones and moves on. But that’s not how real manufacturing works. If you’re in a regulated industry-medical devices, pharmaceuticals, aerospace, or even automotive-you don’t just fix the problem. You corrective actions to make sure it never happens again. And if you don’t? Regulators notice. Customers notice. And your business pays the price.
What’s the difference between a correction and a corrective action?
Let’s say a machine is printing labels too low on a pill bottle. You notice it during a quality check. You stop the line, adjust the sensor, and restart. That’s a correction. It fixes the immediate issue. No paperwork. No meeting. Just a quick fix. But if that same error happens again next week? And again the week after? Now you’re not dealing with a glitch. You’re dealing with a pattern. That’s when you start a corrective action. This isn’t about fixing the label position. It’s about asking: Why did the sensor drift? Was it worn out? Was the calibration schedule ignored? Did someone bypass the alarm? Did training lapse? Corrective actions target the root cause. Corrections fix the symptom. The difference matters because regulators like the FDA and ISO require proof that you’re not just band-aiding problems. You have to prove you’ve removed the cause.The six-step corrective action process
Every serious manufacturer follows a structured six-step process. It’s not magic. It’s methodical. And it’s documented-every step.- Identify the problem - It starts with data. A failed inspection. A customer complaint. A machine alarm. It’s recorded in a system, tagged with time, location, batch number, and operator.
- Evaluate the risk - Not all problems are equal. Is this a safety issue? Could it harm a patient? Is it a minor cosmetic flaw? Risk levels determine how deep you go. A critical issue triggers a full CAPA. A minor one might just get logged.
- Find the root cause - This is where most companies fail. Too many jump to conclusions: "The operator messed up." But operators don’t make mistakes in a vacuum. Tools like the 5 Whys and Fishbone diagrams dig deeper. Why did the operator miss the calibration? Because the schedule wasn’t visible. Why wasn’t it visible? Because the digital work instructions weren’t updated. Why? Because no one checked them after the machine upgrade.
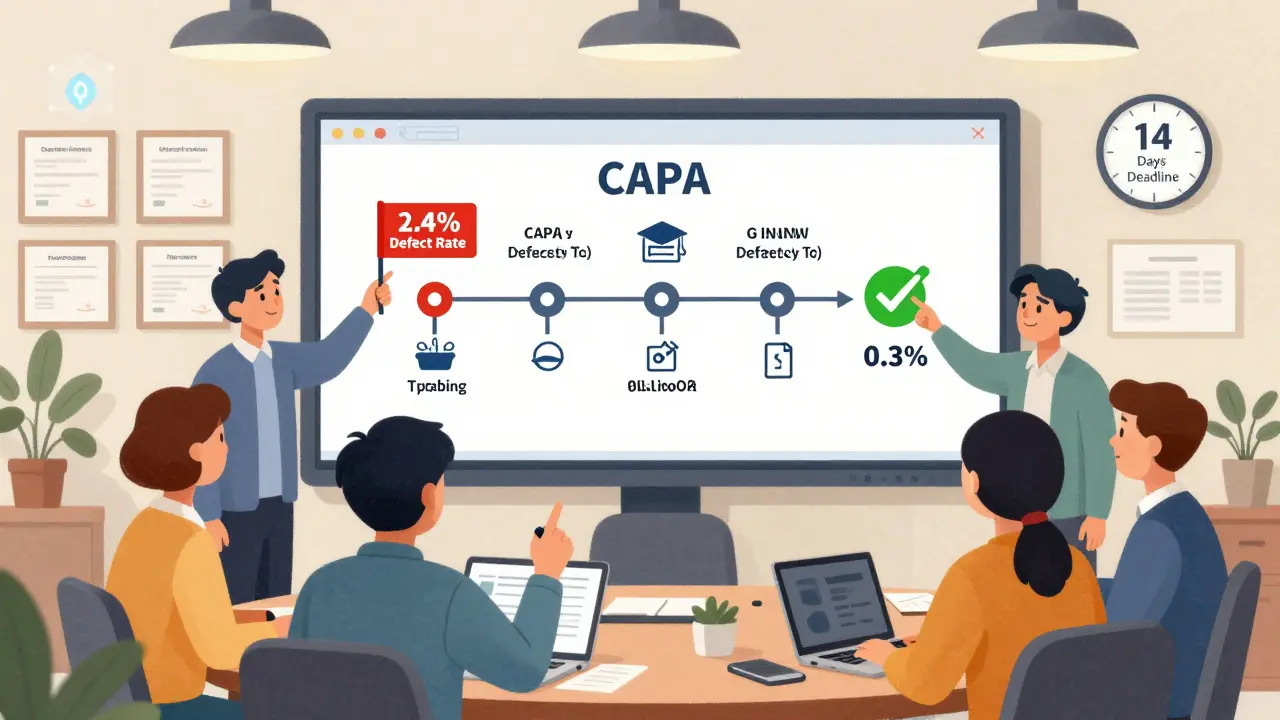
- Plan the fix - You don’t just say "fix the calibration." You write: "Update digital work instructions to include calibration checklist. Train all operators by March 15. Verify compliance with weekly audits. Assign responsibility to Maintenance Supervisor, Sarah Lin. Deadline: 14 days." Specific. Measurable. Assigned.
- Implement the solution - The plan goes live. Training happens. Software gets updated. New checklists are printed. Changes are tracked. No one just "thinks" it’s done. They prove it.
- Verify effectiveness - This is the make-or-break step. You don’t just assume the fix worked. You collect data. You run 30 more units. You track defect rates for the next three production cycles. If the defect rate drops from 2.4% to 0.3%? You’ve succeeded. If it stays at 2.1%? You go back to step three.
Each step leaves a digital trail. Auditors don’t ask if you fixed it. They ask: "Show me the evidence. Show me the before. Show me the after. Show me who signed off. Show me the training records. Show me the test results."
Why do so many corrective actions fail?
The FDA issued 432 warning letters in 2022 related to poor corrective actions. The most common reason? Addressing symptoms, not causes. One medical device maker kept having contamination in their sterile packaging. Their first CAPA? Added an extra wipe-down step. The problem came back. Their second CAPA? Installed a new air filter. Still came back. Only after a third investigation-using a Fishbone diagram-did they find the real cause: a technician was using the same gloves to handle both sterile and non-sterile components. The gloves were the vector. Not the air. Not the wipe-down. Other failures include:- Assigning responsibility to a team instead of a person. "The QA team will handle it." Who? When? What if they’re busy?
- Setting vague deadlines. "Fix it soon." That’s not a deadline. It’s an excuse.
- Skipping verification. "We think it’s fixed." Thinking isn’t proof.
- Not using data. Relying on gut feeling instead of defect trends, control charts, or statistical analysis.
Companies that succeed have one thing in common: they treat corrective actions like engineering problems-not paperwork.
Regulations aren’t optional
If you make medical devices, you’re bound by ISO 13485 and FDA 21 CFR Part 820. If you make drugs, it’s cGMP. These aren’t suggestions. They’re legal requirements. ISO 13485:2016 says corrective actions must "eliminate the causes of nonconformities" and prove they won’t recur. The FDA says you must "verify and validate" your fixes. That means testing. Documenting. Re-testing. If you can’t show proof, your facility can be shut down. In 2023, 28% of all quality system deficiencies found during FDA inspections were related to CAPA. That’s second only to document control issues. It’s not about being perfect. It’s about being systematic.What’s changing in 2026?
The old way of doing CAPA-spreadsheets, printed forms, email chains-is fading. The future is digital and predictive. New ISO 13485 Amendment 1 (effective March 2024) now requires manufacturers to use trending data to spot problems before they become defects. If you see a spike in temperature readings across 10 machines over two weeks? That’s not just data. That’s a warning. You don’t wait for a batch to fail. You act early. AI tools are now being used to analyze production logs and flag anomalies. One company in Wisconsin cut their root cause analysis time from 8 hours to under 4 hours by using AI to cross-reference sensor data, maintenance logs, and operator shifts. Accuracy improved by 37%. The FDA’s Digital Health Innovation Plan is pushing for blockchain-backed audit trails. That means every change to a CAPA document is time-stamped, locked, and traceable. No more backdating. No more deleting. No more "I thought it was approved."
What works in practice?
A small medical device maker in Leeds had a 2.8% defect rate in their implantable sensors. Their old system relied on paper logs. They spent 15% of their quality team’s time just writing CAPAs. No one had time to fix anything. They switched to a cloud-based quality management system with built-in CAPA workflows. Now:- Problems are logged instantly on tablets on the floor.
- Root cause tools are built into the form-5 Whys, Fishbone, all one click.
- Assignments auto-send to the right person.
- Verification metrics are tracked automatically-defect rates, test results, time to closure.
- Documents are signed digitally. No printing. No filing.
Within 18 months, defects dropped to 0.4%. Audit time halved. Regulatory inspections became routine, not terrifying.
It wasn’t the software. It was the structure. The clarity. The accountability.
Is CAPA right for every manufacturer?
Not every factory needs a full CAPA system. A small shop making custom metal brackets for local machinery might only need a simple correction log. If you have 10 defects a year, a 10-page CAPA form is overkill. But if you’re in a regulated industry-or if your customers demand proof of quality-you need more than corrections. You need a system that shows you’re in control. That you’re not lucky. You’re deliberate. The best manufacturers don’t wait for failure. They build systems that catch problems before they become complaints. That’s the real goal of corrective action-not to fix mistakes, but to stop them from ever happening.What’s the difference between corrective action and preventive action?
Corrective action fixes something that already went wrong. Preventive action stops something from going wrong before it happens. For example, if a machine keeps overheating and you fix it after each failure, that’s corrective. If you install a temperature monitor that shuts it down before it overheats, that’s preventive. Both are part of a full quality system, but they serve different purposes.
How long should a corrective action take to complete?
There’s no universal timeline, but most regulated industries expect closure within 30 to 60 days for medium-risk issues. Critical issues affecting safety must be resolved in under 14 days. The key isn’t speed-it’s thoroughness. A rushed CAPA that doesn’t fix the root cause will come back. A slower one that works? That’s a win.
Do I need software to manage corrective actions?
You don’t need software, but you’ll struggle without it. Manual CAPA systems using Excel or paper take 40% longer, increase errors by 30%, and make audits stressful. Digital systems automate reminders, track deadlines, store documents securely, and generate reports. For any manufacturer with more than 50 employees or regulated products, software isn’t a luxury-it’s a necessity.
What happens if I don’t complete a corrective action?
Regulators will find out. During an audit or inspection, they’ll ask for your CAPA records. If they’re incomplete, missing, or ineffective, you’ll get a warning letter. In severe cases, your product can be seized, your facility shut down, or your certification revoked. Even if you’re not regulated, customers will lose trust. One major automotive supplier lost a $12M contract in 2023 because their CAPA logs were inconsistent.
Can one person handle all corrective actions?
No. Corrective actions require cross-functional input. The quality team identifies the issue, engineering identifies the technical fix, production implements it, training ensures people know how to do it right, and management verifies it works. Trying to do it alone leads to blind spots. That’s why ISO 13485 requires cross-functional teams for every CAPA.




Bobbi-Marie Nova, January 18, 2026
So you're telling me the FDA cares more about paperwork than actual patient outcomes? Cool. I'll just keep my fingers crossed the guy who printed the labels didn't just have a bad day.
Meanwhile, my cousin works in a lab where they still use clipboards. He says they're faster and less likely to crash. Just saying.
Allen Davidson, January 18, 2026
This is actually one of the most clear-headed takes on CAPA I've seen in years. Too many companies treat it like a box to check instead of a system to live by.
The part about assigning responsibility to a person-not a team-is gold. If no one owns it, it dies. Simple as that.
And the AI example? That’s the future. Not magic, just smart data use. We’ve seen similar results in our plant-cut analysis time in half and caught three potential failures before they even happened. No one’s impressed until they’re audited. Then they wish they’d listened sooner.
john Mccoskey, January 19, 2026
Let’s be honest-this entire CAPA framework is a bureaucratic illusion designed to make managers feel in control while the actual production floor continues to rot from within.
The 5 Whys? A parlor game for people who think causality is linear. Reality is chaotic. Operators don’t fail because training was ‘inadequate’-they fail because they’re overworked, underpaid, and told to ‘just make it work’ while the VP takes a vacation to Bali.
Root cause analysis is a myth. The real root cause is capitalism. The system rewards compliance over competence. You can document your way to a 0.3% defect rate, but if the machine is 17 years old and nobody has the budget to replace it, you’re just painting over rust.
And don’t get me started on blockchain audit trails. That’s not innovation-that’s digital feudalism. Someone’s going to get rich off this, and it won’t be the guy who’s actually fixing the machines.
Ryan Hutchison, January 19, 2026
America invented quality control. We built the world’s best factories. Now we outsource everything and wonder why our standards are crumbling.
This whole CAPA thing? It’s not about software or AI. It’s about discipline. You want to fix problems? Stop outsourcing to India and China. Stop hiring people who don’t care. Stop treating engineers like disposable labor.
Our grandparents didn’t need cloud-based QMS systems. They had pride. They had standards. They didn’t need an app to tell them not to use the same gloves for sterile and non-sterile stuff.
We lost the culture. Now we’re trying to code our way out of it. That’s not innovation. That’s surrender.
Melodie Lesesne, January 20, 2026
I love how this breaks it down without the jargon. We rolled out a similar system last year and honestly? It’s been a game changer.
Before, people avoided reporting issues because they knew it meant a 3-week paperwork marathon. Now they log it on their phone while waiting for the oven to heat up. Simple. Fast. No shame.
And yeah, the defect rate dropped. But more importantly, people feel heard. That’s the real win.
Corey Sawchuk, January 20, 2026
the part about verification being skipped is so real
we had a case last year where someone said 'it's fixed' and moved on
two weeks later same problem
turns out the new checklist was never printed
no one checked
no one cared
just another ghost in the machine
Rob Deneke, January 21, 2026
Man I wish more people understood the difference between correction and corrective action
I’ve seen teams fix the same sensor issue 7 times because they kept calling it a ‘quick fix’
you don’t fix a leak by mopping the floor
you find the pipe
and yeah the software helps but the culture matters more
if your team’s scared to speak up you’re already dead
just sayin’
Chelsea Harton, January 22, 2026
corrective action is just fancy talk for ‘stop being lazy’
and yes the software helps but the real fix is paying people enough to care